 Research
Publications
Members
Contact
Research
Publications
Members
Contact
The cell cycle dependent chromatin dynamics and how it helps to maintain genome stability
At a glance:
The dynamics of chromatin, and in particular the centromere, is central in dividing cells. Defective chromatin regulation can result in genome instability and unsolicited proliferation, both hallmarks of tumor cells.
More specifically, we are interested primarily in three aspects of chromatin modifications: what happens when normal cells are taken out of the cell cycle during ageing (1) and after DNA damage (2) and what are the routes that can go wrong and allows a cell to continue to divide when it should not, resulting in a person developing cancer (3). In the lab we apply high throughput molecular and cellular techniques, such as sequencing of RNA (coding/non-coding) and chromatin immunoprecipitation (ChIP), and automated microscopy. In general, by combining model organism Schizosaccharomyces pombe with human material, cell lines and primary material (healthy and derived from AML patients), we try to get to mechanistic detail of clinically relevant questions. S. pombe genetics and genomics let us rapidly and precisely dissect causal relationships. We focus on highly conserved pathways and parallel work in human cells maintains relevance for human health.
Background:
Centromeres: specialized and dynamic chromatin
The centromere is the part of a chromosome that links sister chromatids. The kinetochore forms at the centromere chromatin and during mitosis, microtubuli attach to the centromere via the kinetochore to distribute the sister chromatids in the daughter cells (Figure 1). Most eukaryotes have regional centromeres that occupy 40 kb – 100 kb of the chromosome. Rather than being specified by the DNA sequence, centromeres are defined epigenetically by the presence of centromeric histone H3 variant CENP-A in the central domain. The deposition of CENP-A is required for kinetochore assembly. Centromeres in most eukaryotes have two distinct domains: the centromeric CENP-A chromatin and the flanking pericentric heterochromatin. Both domains are needed for centromere functions. The centromeric structure differs largely between organisms, e.g. in S. pombe and mouse, the centromeric and pericentromeric domains are clearly defined and associated with specialized DNA sequences. In other species, including human, the centromeric DNA is mainly made up of repetitive a-satellite sequence (ALR) and do not distinguish between the two types of centromeric chromatin, although the compartments are distinct.
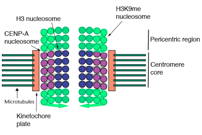 Centromere transcription in actively dividing cells
Centromere transcription in actively dividing cells
Active centromeric central cores are transcribed by Pol II in a H2Bub1 dependent manner. We have studied this non-coding transcription in both S. pombe and human cells. For the formation of pericentric heterochromatin in S. pombe, a role for non-coding RNA is well established since the key discovery of RNAi in heterochromatin formation.
Research questions:
Three main areas are under focus in our group:
We and others have clarified the role of centromeric transcription in S. pombe. However, the centromere transcription situation in mammalian cells is less clear. The centromere sequence composition may offer explanations to the possible discrepancies between model organisms and human. Whereas in S. pombe, transcription of the pericentric DNA induces RNAi-driven heterochromatin formation, the same function in human cells would need further distinction than available from the transcript sequence to avoid silencing of the central domain.
Centromere inactivation occurs naturally when cells stop dividing. This happens during senescence when an organism ages. Also, when dicentric chromosomes arise, silencing of one of the centromeres provides a means for a cell to survive. It is known that this occurs by down-regulation of CENP-A.
Normally in adult somatic cells, centromeres are not transcribed, or they are transcribed at low levels. However, the centromeres of tumor cells often have increased levels of transcription. Disabling the process of centromere inactivation is one way we hypothesis a tumor cell can escape cell cycle arrest and continue to proliferate. We aim to decipher mechanisms behind centromere maintenance and inactivation.
Methods:
Many of the results from the lab are generated through large data sets and analysis of the functional aspects of the genome. This work involves the use of genomic data sets to understand the gene and protein functions and interactions, in short: functional genomics.
Development of computational analysis tools:
To detect exact positions of modifications, chromatin immunoprecipitation (ChIP)-seq (or ChIP-chip) is used. To allow extensive analysis we are developing computational tools that specifically suits our needs.
In collaboration with the lab of Karl Ekwall, we are setting up a Positioning Database and Analysis Tool (Podbat). In Podbat, users can upload chromosome position or RNA expression data which will be mapped onto the genome and visualized. Specific regions of interest can be computationally identified and the deviation between experimental conditions can be quantified. Comparisons between different datasets are relatively easy to make and lists of genes or regions can be queried for correlations between experiments or enrichment of functional categories.
Podbat is a Java-based tool that is still under development but can be downloaded from www.podbat.org. It is described in Sadeghi et al, 2011.
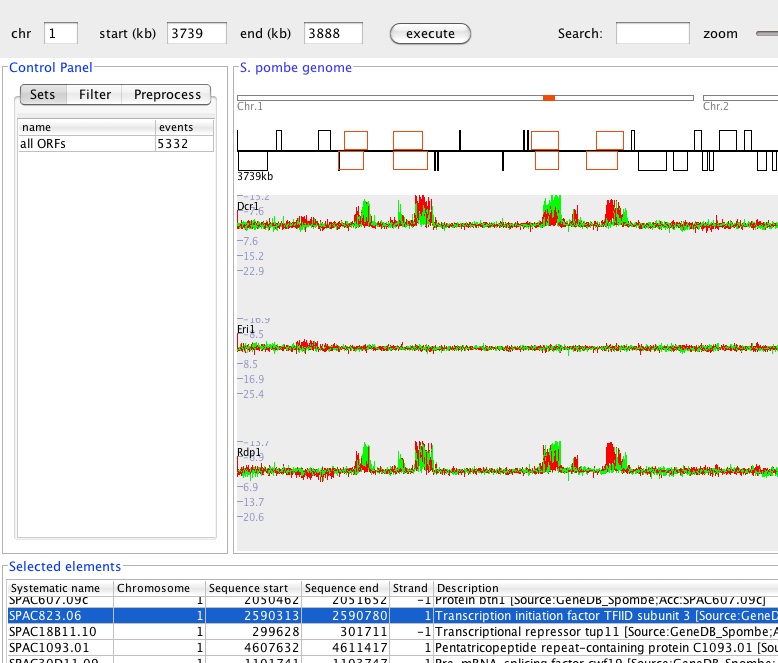
Figure 2: Screenshot of the Podbat tool with a RNA expression dataset loaded revealing expression of small RNAs as the outer repeats of the centromere (Ingela Djupedal).